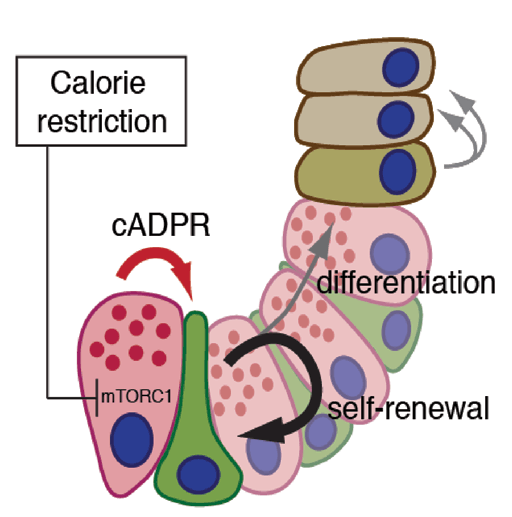
Since ISCs, like all adult stem cells, posses the ability to self-renew (i.e. generate daughter stem cells) and the capacity for multipotent differentiation (i.e. generate lineage-committed progenitors and ultimately all mature tissue-specific cell types), they likely play an important role in remodeling the intestine in response to diet-induced physiologies.
A majority of ISCs express the G protein-coupled receptor 5 (Lgr5) and reside at the bottom of intestinal crypts nestled between Paneth cells. These cells constitute a component of the stem cell cellular neighborhood or “niche,” and elaborate myriad growth factors and cues necessary for the maintenance of ISCs. The Yilmaz lab is working on elucidating the molecular mechanisms underpinning the interaction between ISCs and Paneth cells in low-calorie diets such as calorie restriction and pro-obesity diets such as high-fat diets. By comparing how ISCs and their surrounding cells adapt to diverse nutrients, they will gain a deeper understanding of how the intestine integrates physiology with its growth and why some diets reduce or increase the risk of colon cancer.
Also, his lab has developed numerous tools and techniques to better model colon cancer using murine and patient-derived tissues. In one approach, CRISPR/CAS9 genome edited intestinal cancer organoids (that is, 3-D primary “mini-intestinal cancers”) are endoscopically transplanted into the colons of recipient mice. In a complementary approach, viral particles that contain CRISPR/CAS9 components are endoscopically directed to the intestinal mucosa to induce in situ colonic tumors. With such methods, it now possible to robustly model the various steps of intestinal tumorigenesis from early precursor lesions to liver metastasis. The development of such tools will enable them to dissect how different dietary states, aging, and the gut microbiome influence tumor initiation, growth, metastasis, and drug resistance.
Figure 1. Dietary Regulation of Stem Cells in Tissue Homeostasis:
(adapted from Yilmaz et al Cell Stem Cell 2014)
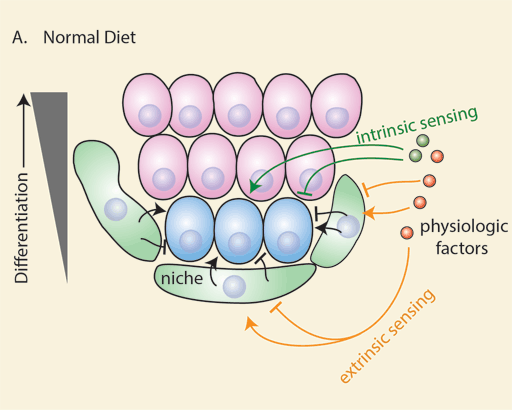
(A) Intrinsic (dark green) and extrinsic (orange) diet-sensing mechanisms integrate diet-induced physiology with tissue homeostasis. Stem cells (blue) and their niche (green) sense physiologic cues such as hormones, growth factors, and nutrients to dynamically alter the production of differentiated cells (pink).
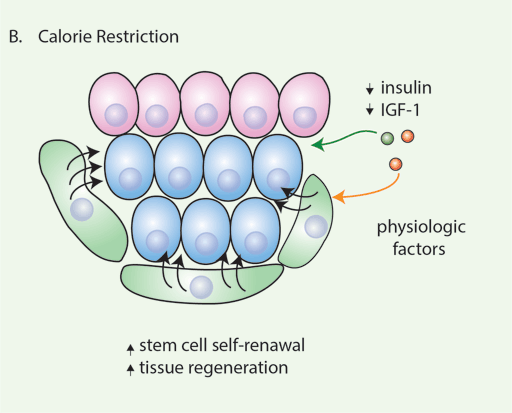
(B) Calorie restriction boosts regeneration in diverse tissues by increasing stem cell numbers and function. Niche-derived signals mediate some of the response of calorie restriction on stem cells.
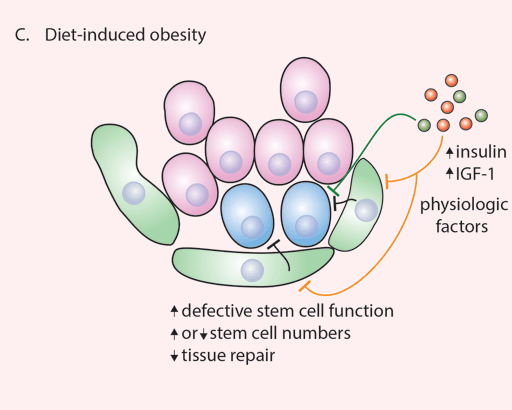
(C) Diet-induced obesity is associated with an abundance of nutrients, growth factors, and hormones that eventually leads to physiologic disequilibrium, including insulin resistance, diabetes, and metabolic syndrome. This state reduces tissue repair, in part due to dysfunction of stem cells, their niches, or both.
Figure 2. Diet and Cancer Initiation:
(adapted from Yilmaz et al Cell Stem Cell 2014)
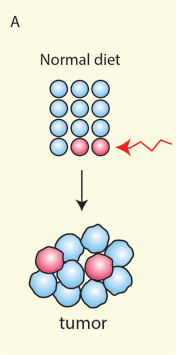
(A) In tissues that follow a stem cell paradigm, stem cells (red) acquire early oncogenic events (red arrow) that lead to transformation and tumor formation.
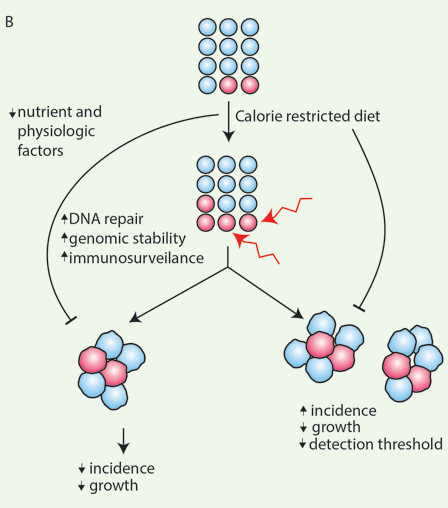
(B) Calorie restriction augments stem cell numbers and function in diverse tissues and is proposed to have antitumor initiation and growth effects. If stem cell numbers increase with calorie restriction and they undergo some of the early changes that give rise to tumors, calorie restriction may potentially increase tumor incidence. It is possible that autonomous and nonautonomous protective mechanisms are activated in stem cells with calorie restriction, whichneutralize the effects of a larger, more robust stem cell pool. Another possibility may be that the antigrowth effects of calorie restriction on tumor growth mask its effects on initiation. Tumors arising in calorie restriction may remain below detection threshold because they are small in size.
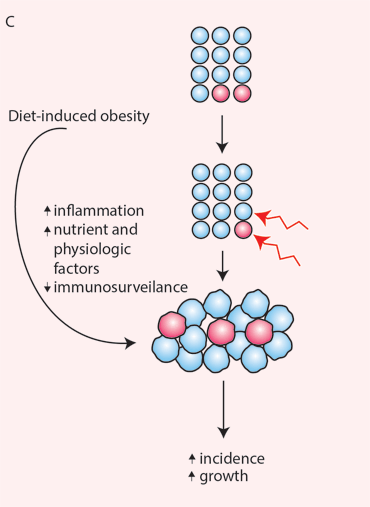
(C) Diet-induced obesity has untoward effects on tissue repair and cancer incidence. Although stem cell numbers can decrease with chronic obesity, the susceptibility of differentiated cells to undergo transformation can also increase as has been noted to occur with inflammation. In this case, early oncogenic events can occur in stem cells and differentiated cells, effectively increasing the pool of cells that can undergoearly transformation. Surplus growth factors, nutrients, and hormones then drive tumor progression and growth.
Figure 3: Calorie Restriction